Science experiments are not only educational but also incredibly fascinating, offering a hands-on approach to understanding complex scientific principles. Through engaging and interactive demonstrations, these experiments bring abstract concepts to life, making learning both enjoyable and memorable.
From creating explosive reactions to observing the behavior of fluids and exploring the mysteries of magnetism, these experiments promise to captivate the imagination and spark curiosity. So, roll up your sleeves, gather your materials, and prepare to embark on an exciting journey of discovery through the world of science experiments.
Elephant Toothpaste
This experiment visually demonstrates an exothermic chemical reaction, primarily involving the decomposition of hydrogen peroxide (H2O2). When hydrogen peroxide comes into contact with yeast, the yeast acts as a catalyst, speeding up the breakdown of hydrogen peroxide into water (H2O) and oxygen (O2) gas. The rapid release of oxygen gas creates bubbles when it interacts with dish soap added to the mixture, resulting in a large, foamy substance that erupts out of its container, resembling toothpaste being squeezed from a tube. This foam is safe to touch but can be warm due to the exothermic reaction releasing heat. The experiment is a vivid example of catalysis, showing how a catalyst can accelerate a chemical reaction without being consumed in the process.
Non-Newtonian Fluid
This experiment involves mixing cornstarch and water to create a mixture that demonstrates properties of both solids and liquids, depending on the force applied. This substance is known as a non-Newtonian fluid, specifically, a dilatant material that becomes more viscous (solid-like) under stress. When you slowly dip your hand into it, the mixture feels like a liquid, but when you punch or apply sudden force, it hardens and resists the motion. The scientific principle behind this behavior is shear thickening, where the particles within the mixture lock together under pressure. This experiment is an engaging way to explore complex fluid dynamics and the behavior of materials under different conditions.
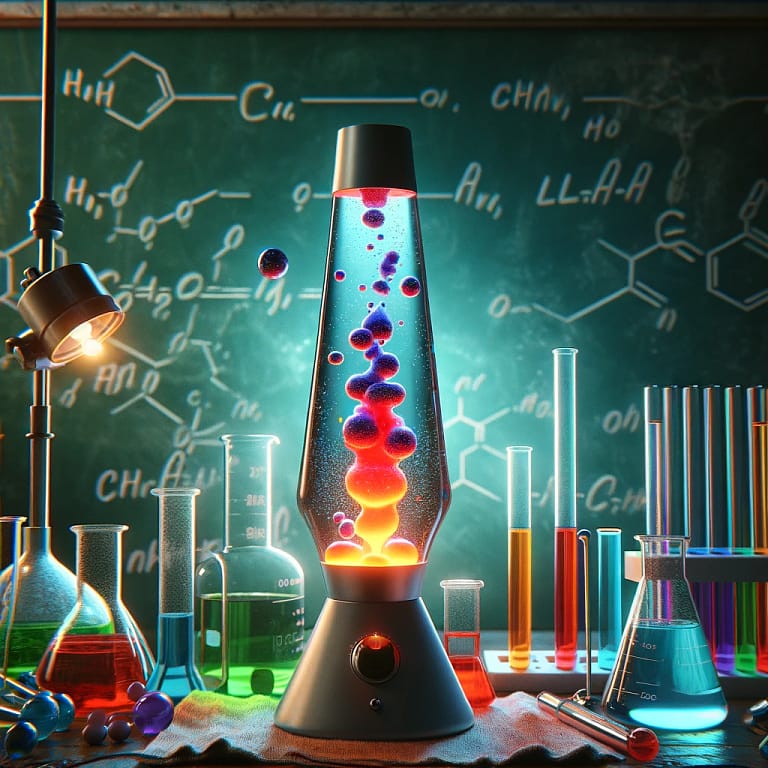
Homemade Lava Lamp
Creating a homemade lava lamp demonstrates principles of density and solubility. The experiment starts with filling a clear container with two immiscible liquids: water and vegetable oil. Since oil is less dense than water, it floats on top. Adding food coloring allows for visualization as the drops pass through the oil and mix with the water. Introducing an effervescent tablet (such as Alka-Seltzer) into the container causes it to dissolve in the water, creating carbon dioxide gas. As the gas bubbles form, they attach to the colored water droplets, causing them to rise through the oil. Once the gas escapes at the surface, the density of the water causes it to sink again. This cycle creates the lava lamp effect, illustrating the concepts of density, solubility, and the behavior of gases in liquids.
Magnetic Slime
The creation of magnetic slime involves mixing a typical slime recipe (using borax and glue) with iron filings. The iron filings are ferromagnetic, meaning they are attracted to magnets and can be manipulated by magnetic fields. When a strong magnet is brought close to the slime, the iron filings within the slime align with the magnetic field lines, causing the slime to stretch, spike, or move towards the magnet. This experiment not only explores the properties of polymers and viscosity but also introduces the concept of magnetism and magnetic fields. The slime’s behavior when exposed to a magnetic field provides a tangible demonstration of how ferromagnetic materials respond to magnets, making it a fascinating exploration of both chemistry and physics.
Rainbow in a Glass
This experiment showcases the principles of density and stratification. By carefully layering sugar solutions of different concentrations (each colored with food dye) in a transparent glass, distinct layers form due to variations in density. The densest solution settles at the bottom, while the least dense solution stays at the top. The different densities prevent the liquids from mixing thoroughly, creating a visually appealing rainbow effect in the glass. This demonstration vividly illustrates how substances of different densities can form layers when combined and left undisturbed, providing a tangible example of stratification commonly observed in nature, such as in oceans and lakes.
Copper-Plated Coins
This experiment demonstrates electrochemistry principles by copper-plating coins using simple household materials. First, tarnish and oxides are removed from the surface of pennies by soaking them in a solution of vinegar (acetic acid) and salt (sodium chloride). Then, the cleaned pennies are placed in a separate solution containing copper-coated items, such as screws or wire. Through a process called electrolysis, an electrochemical reaction occurs, where copper ions from the copper-coated items are transferred onto the surface of the pennies. This results in the pennies becoming plated with a thin layer of copper, giving them a shiny appearance. The experiment highlights how electricity can be used to drive chemical reactions and deposit metals onto surfaces, providing a practical demonstration of electroplating and the principles of electrochemistry.
Balloon and Static Electricity
This experiment illustrates the principles of electrostatics by demonstrating how static electricity can cause objects to attract or repel each other. By rubbing a balloon against hair or fabric, the balloon becomes negatively charged due to the transfer of electrons. This excess negative charge causes the balloon to stick to walls or other surfaces temporarily. Additionally, the charged balloon can also bend water streams or attract small pieces of paper, showcasing the electrostatic forces at play. The experiment serves as a tangible demonstration of how objects can become charged through friction, leading to interesting interactions with other charged or neutral objects based on the principles of electrostatic attraction and repulsion.
DIY Volcano
This classic experiment simulates a volcanic eruption and demonstrates the principles of chemical reactions. A mixture of baking soda (sodium bicarbonate) and vinegar (acetic acid) is combined inside a constructed clay volcano. When these two substances react, carbon dioxide gas is rapidly produced, creating a frothy eruption that resembles lava flowing from a volcano. The chemical reaction between the acidic vinegar and the basic baking soda produces carbon dioxide gas, which expands and creates the bubbling effect seen during the eruption. This experiment provides a hands-on way to explore chemical reactions, particularly acid-base reactions, and introduces the concept of gas production during chemical change, making it both educational and entertaining.
Homemade Ice Cream in a Bag
This experiment demonstrates physical changes and the phenomenon of freezing point depression while making delicious homemade ice cream. A mixture of milk, sugar, and vanilla extract is placed in a small sealed bag, while a larger bag is filled with ice and salt. The addition of salt to the ice lowers its freezing point, creating a brine solution that absorbs heat from the milk mixture. As a result, the milk mixture cools down rapidly, causing the water molecules to freeze and solidify into ice cream. By shaking the bags vigorously, the ice cream mixture freezes evenly, resulting in a creamy texture. This experiment provides a fun and tasty way to learn about physical changes, freezing point depression, and the effects of salt on freezing temperatures.
Exploding Baggie (Chemical Reaction)
In this experiment, a mixture of vinegar and baking soda is sealed inside a plastic baggie. The baggie is quickly sealed shut and then shaken to ensure thorough mixing of the two substances. As the vinegar (acetic acid) reacts with the baking soda (sodium bicarbonate), carbon dioxide gas is rapidly produced. The pressure from the gas buildup causes the baggie to expand until it eventually bursts, resulting in a small but impressive “explosion.” This experiment vividly demonstrates the principles of chemical reactions, specifically acid-base reactions, and the production of gas as a byproduct.
Disappearing Eggshell (Acid-Base Reaction)
This experiment involves soaking a raw egg in vinegar for a period of several days. Over time, the vinegar (which is acidic) reacts with the calcium carbonate in the eggshell, causing it to dissolve. The calcium carbonate breaks down into calcium ions, carbon dioxide gas, and water. As a result, the eggshell gradually dissolves, leaving behind a transparent, rubbery egg membrane. This experiment provides a tangible demonstration of acid-base reactions and the effects of acidic substances on calcium-based materials.
DIY Cartesian Diver (Buoyancy and Pressure)
A Cartesian diver is a classic science toy that demonstrates principles of buoyancy and pressure in fluids. To create a DIY Cartesian diver, fill a plastic bottle with water and insert a small, hollow object such as an eyedropper or pen cap partially filled with water. When the bottle is squeezed, the pressure increases, causing the air inside the diver to compress and the density of the diver to increase. As a result, the diver sinks. When the pressure is released, the air inside the diver expands, decreasing its density, and causing it to float back to the surface. This experiment provides a hands-on way to explore the concepts of buoyancy, pressure, and the behavior of objects in fluids.